FDA approves first topical minocycline Foam 1.5% ZILXI for the treatment of inflammatory rosacea. Minocyclin is a tetracycline-class drug that was originally approved as an oral formulation for the treatment of acne vulgaris but now being developed into topical formulation to treat both acne vulgaris and rosacea. Previously on Oct 2019 same formulation in the name of Amzeeq 4% topical foam was approved for the treatment of acne.
ZILXI, developed as FMX103 by Menlo’s wholly-owned subsidiary Foamix Pharmaceuticals Ltd. (“Foamix”), is the first minocycline product of any kind to be approved by the FDA for use in rosacea.
“This approval is welcome news for clinicians and patients who seek novel options for this difficult to treat skin disorder,” said David Domzalski, Chief Executive Officer of Menlo. “ Minocycline Foam ZILXI 1.5% is a potential turning point in rosacea treatment, providing millions of people with a new treatment option that is well-tolerated and effective.”
Rosacea is a diverse skin condition that most commonly presents with symptoms such as deep facial redness, spider veins (telangiectasia) and acne-like inflammatory lesions (papules and pustules).
Minocycline is one of several broad-spectrum antibiotics known as tetracyclines with anti-inflammatory properties; their use in some patients is limited due to systemic side effects when taken orally. In ZILXI, Menlo has once more leveraged its proprietary Molecule Stabilizing Technology (MST™) platform to effectively deliver minocycline in a foam-based vehicle.
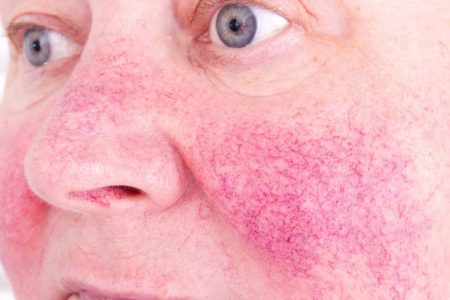
“This is the only product containing minocycline approved by the FDA for rosacea,” said Iain Stuart, PhD, Chief Scientific Officer of Menlo. “The availability of a novel topical formulation of this molecule underscores our efforts to provide innovative treatment options for patients who suffer from difficult to treat skin conditions.”
The FDA approval of ZILXI is primarily supported by data from two clinical trials in 1,522 patients 18 years of age and older. In each 12-week multicenter, randomized, double-blind, vehicle-controlled trial, subjects with inflammatory lesions of rosacea were treated once daily with ZILXI or vehicle. No other topical or systemic medication affecting the course of inflammatory lesions of rosacea was permitted for use during these trials.
The co-primary efficacy endpoints were (a) the absolute change from baseline in inflammatory lesion counts at Week 12 and (b) the proportion of subjects with treatment success at Week 12 defined as an IGA score of 0 (“clear”) or 1 (“almost clear”), and at least a two-grade improvement (decrease) from baseline at Week 12. ZILXI met both co-primary endpoints in each clinical trial, demonstrating statistically significant improvements in inflammatory lesion count and Investigator Global Assessment (IGA) treatment success.
No treatment-related serious adverse events were reported. The most common adverse reaction reported by ?1% of subjects treated with ZILXI and more frequently than in subjects treated with vehicle was diarrhea (1% vs. 0%), respectively.
Menlo anticipates having ZILXI available for prescribing by 4th quarter of this year.
News Source: Menlo Therapeutics




Leave a comment